Chemical Reactions - Analytical Chemistry: An Indian Journal
Analytical Chemistry: An Indian Journal is a peer-reviewed, open-access academic magazine that disseminates unique, original scientific writings based on the various disciplines of agriculture and medicine to talk about a variety of brand-new difficulties in analytical chemistry.
The Analytical Chemistry: An Indian Journal is committed to the timely publication of significant original research in the fundamental theory, practice, and application of analytical and bioanalytical science. This includes the miniaturization of analytical systems, bioanalyses, chromatography, mass spectrometry, electrophoresis, electrochemistry, sampling, and sample handling, as well as atomic and molecular spectroscopy. Each submission will go through a thorough peer review process, with consideration given to the quality, originality, and variety of reader interests. The journal maintains its scientific standing by presenting the most important current discoveries across all areas of analytical chemistry.
Recently In our upcoming issue, we have published an article entitled “Elimination of Methyl Orange Using a Novel Anionic Adsorbent Prepared by Radiation Grafting Followed by Chemical Treatment” by one of the eminent author “Nazia Rahman’’ Nuclear and Radiation Chemistry Division, Institute of Nuclear Science and Technology, Atomic Energy Research Establishment, Bangladesh Atomic Energy Commission, Dhaka, Bangladesh.
This article is about Glycidyl Methacrylate (GMA) was grafted onto non-woven polyethylene textiles using a -ray initiator, and then the amine was functionalized. Investigated was the relationship between amination yield and grafting intensity. A lot of research was done on the functionalized GMA-g-non has woven PE textiles' ability to absorb the anionic dye Methyl Orange (MO) from an aqueous solution. On MO adsorption aptitude, experiments were conducted to determine the effects of changing the adsorption environment's contact time, temperature, pH, and initial metal ion concentration. A contact period of 48 h, the initial metal concentration of 500 ppm, pH of 1.759, and a temperature of 80oC were found to be the best conditions for maximizing MO adsorption. Langmuir isotherm theory was taken into consideration in order to comprehend the equilibrium between MO and the absorbent system.
Following weighing, a Co-60 gamma radiation source was used to irradiate the non-woven PE textiles. 30 kg of material received a total dosage of radiation, which was applied at room temperature. PE films were preserved in dry ice after irradiation in order to preserve the free radicals. The monomer solution contains distilled water, 5% glycidyl methacrylate, and 0.5% Tween-20 (as an emulsifier). The emulsion solution was placed into a gas passage jar after an hour of stirring. To remove dissolved oxygen, argon gas has flowed through the monomer solution for an hour. The de-aerated monomer solution was immediately put into a glass tube containing pieces of nonwoven PE textiles that had previously undergone radiation treatment.
At 25°C, 50 ml of aqueous solutions of MO were added to the aminated PE films. On MO adsorption aptitude, experiments were conducted to determine the effects of changing the adsorption environment's contact time, temperature, pH, and initial metal ion concentration. HCl and NaOH solution were used to adjust the pH of the solutions. A UV spectrophotometer was used to measure the MO concentrations in the solutions both before and after the adsorption progression.
Investigations were conducted on the amine functionalization of GMA-g-non woven PE film and glycidyl methacrylate grafting caused by gamma radiation on non-woven polyethylene fabric. FTIR and SEM techniques were used to successfully characterize the adsorbent. The adsorption of MO from aqueous solution onto the amine-functionalized GMA-g-non woven PE film was investigated. We carefully examined how adsorption responds to changes in contact time, initial metal ion concentration, pH, and temperature. It was discovered that contact period of 48 h, the initial metal concentration of 500 ppm, pH 1.759, and temperature of 80oC were the optimal conditions that produced the greatest MO adsorption.
The results of the experiments showed that as the MO solution's temperature rose, so did the amount of adsorption. At low temperature (0oC), the amount of adsorption was 17.85 mg/g, while at room temperature (25oC), the amount of adsorption was 20 mg/g after 5 h at 500 ppm MO concentration. The amount of adsorption was 36.94 mg/g at 80oC.
To achieve the equilibrium between MO and the absorbent system, the Langmuir isotherm model was taken into consideration. The Langmuir isotherm model's ideal fitting suggested that MO adsorption be limited to a single molecular layer. The Langmuir isotherm model was used to calculate the maximum monolayer adsorption capacity, which was 60.60 mg/g. With the use of pseudo-first-order and pseudo-second-order models, the adsorption kinetics was studied. The pseudo-second-order model proved successful in resolving the MO adsorption. Successful MO desorption followed by the reuse of the recovered adsorbent fabric examined the potential for the adsorbent's practical use in the future.
An interested author can submit their unpublished work in the form of a Research Article, Case report, etc.
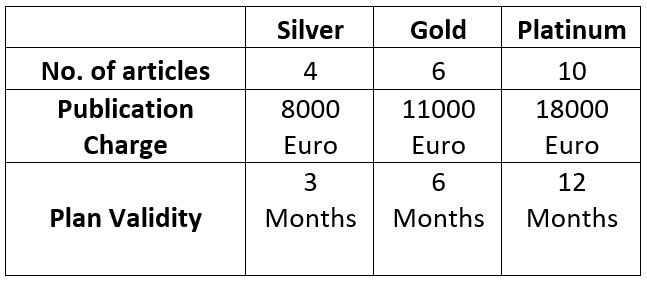
As we know Christmas is near and we will be celebrating it, we present you with an amazing offer for a Christmas celebration in this we are providing a membership plan for eminent authors who will dedicate themselves to our research work.
Note: Our journal’s normal APC is 2300 Euro, but during this Christmas celebration we are giving concessions on APC details as follows:
Submission link- https://www.scholarscentral.org/submissions/analytical-chemistry-an-indian-journal.html
Whatsapp No.- +443308089004
Email Id:- janalchem@theresearchpub.com




Comments
Post a Comment